GEM platform
(RE)PROGRAMMING
THE MICROBIOME
Bringing gene editing to the microbiome
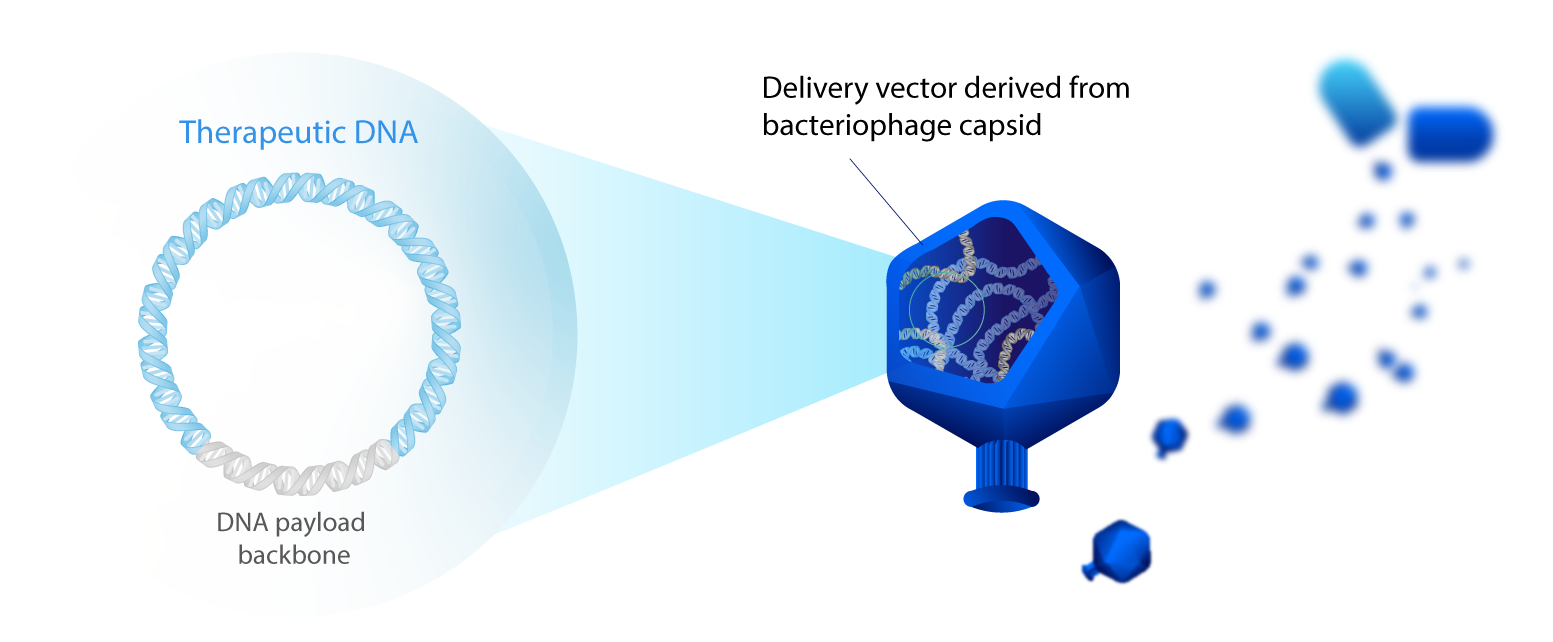
Eligo develops first-in-class proprietary modalities that enable the delivery and expression of therapeutic DNA in target bacterial populations of the microbiome. This technology allows for the first time the in-situ modulation of the microbiome’s composition and function to address human disease with an unprecedented precision.
1/ SSAM technology
Sequence-Specific Anti-Microbials
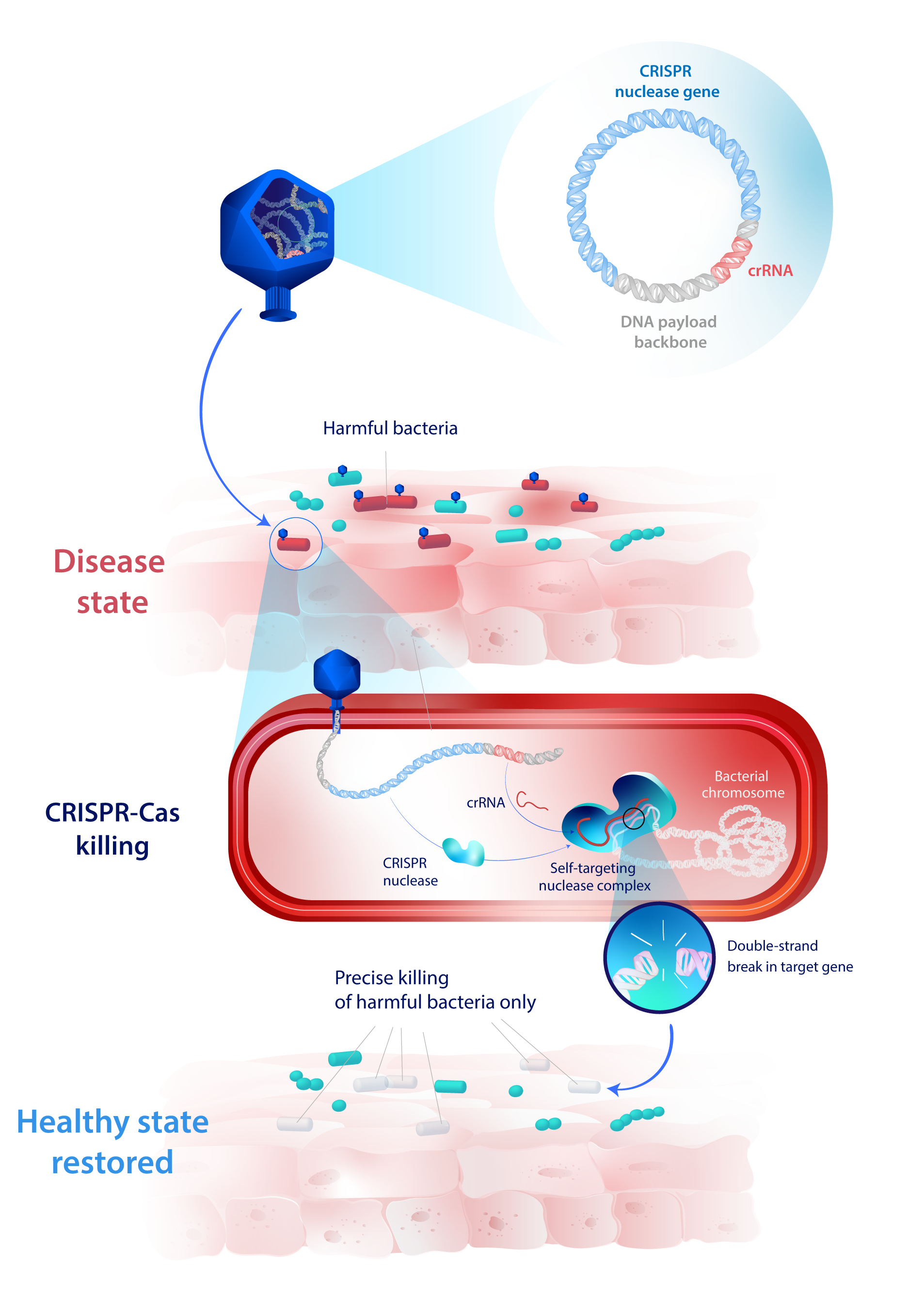
Armed with a CRISPR system, our Eligobiotics® become highly precise antimicrobials. They kill only the harmful bacteria by creating lethal DNA breaks in target sequences of their genome, while leaving the beneficial bacteria completely intact.
—
Therapeutic payload: exogenous CRISPR-Cas nuclease gene and its corresponding guide RNA
MoA: Once the DNA payload is injected in the bacteria, the nuclease is expressed and its corresponding guide RNA will guide it toward bacterial genomic sequences homologous to the guide RNA. If such sequences are present, the nuclease will create targeted DNA double-strand breaks, leading to the death of the bacteria. If these sequences are absent from the bacteria, the nuclease won’t create any double-strand breaks, and the bacteria will be left intact.
This strategy enables the engineering of the microbiome composition with unprecedented precision, killing only the strains harboring genomic sequences targeted by the nuclease.
2/ GEM technology
Genome Editing of the Microbiome
Armed with a gene-editor system, our Eligobiotics® can genetically modify bacteria with nearly 100% efficiency directly in vivo.
—
Therapeutic payload: gene editor and its corresponding guide RNA(s)
MoA: Once the DNA payload is injected in the bacteria, the gene editor is expressed and its corresponding guide RNA will guide it toward bacterial genomic sequences homologous to the guide RNA. If such sequences are present, the editor will modify a nucleotide to another nucleotide type, leading to the durable genomic modification of the target bacterial population.
This technology provides scientists with a novel strategy to better understand how genes from our microbiome drive disease, as well as creates new opportunities for the development of innovative therapies that can inactivate disease-driving bacterial genes.
3/ FAME technology
Function Addition to the MicrobiomE
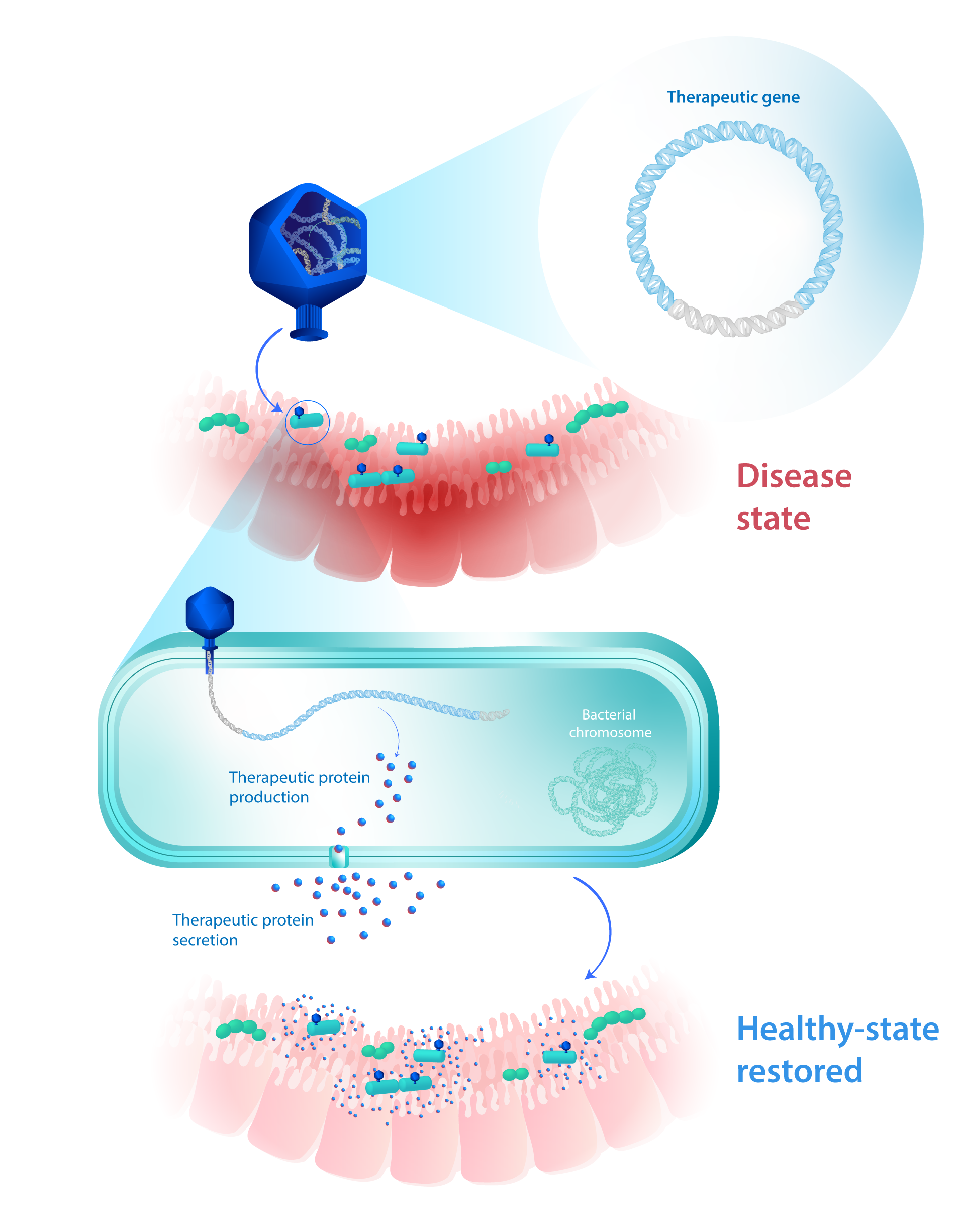
Our Eligobiotics® can be used to leverage microbial populations to transiently produce, display, or secrete an exogenous gene or set of genes to have a therapeutic effect on the host.
—
Therapeutic payload: single gene cassette/operon or multiple gene cassettes/operon(s) – up to 50kb
MoA: Once the non-replicative DNA payload is injected in the bacteria, the gene(s) will be efficiently expressed to have the desired effect on the host.
This strategy enables the engineering of the microbiome function with unprecedented modularity, either to complement the absence of a beneficial gene from the microbiome or to add a novel therapeutic function.
Publications
Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials
2014 – Nature Biotechnology
Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases
2014 – Nature Biotechnology